Abstract
Hematopoietic Stem Cells (HSCs) are the most primitive cells of the hematopoietic system, defined by their ability to sustain lifelong hematopoiesis and differentiate to all mature hematopoietic lineages. HSC transplant (HSCT) is the first-line treatment for many patients diagnosed with a serious hematological disease, however HSCs are an exceedingly rare cell population that are challenging to expand ex vivo. Generating functional HSCs ex vivo from reprogrammed somatic cells or pluripotent stem cells (PSC) is a promising alternative. However, a gap remains in the knowledge of the developmental pathways regulating HSC ontogenesis, making it challenging to reproducibly engineer bona fide HSCs. Hematopoietic stem and progenitor cells (HSPCs) originate from an endothelial-to-hematopoietic transition (EHT) during embryogenesis. EHT is a step-wise process initiating when a specialized subset of endothelial cells (EC), termed hemogenic endothelial cells (HEC) undergo dyshesion from the endothelial bed and form intra-aortic hematopoietic clusters within the major arteries of the embryo. The processes driving the specification of these HEC rather than a vascular fate is not well understood, despite representing a critical need to replicate EHT in vitro.
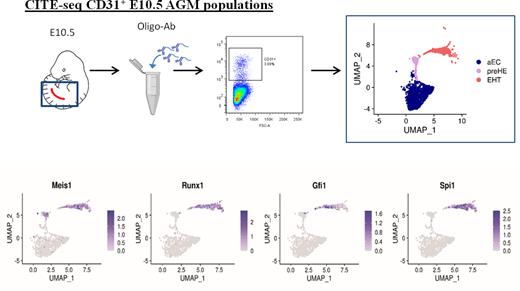
To define factors in the endothelium that prime EC towards a hemogenic phenotype, we performed Cellular Indexing of Transcriptomes and Epitopes by Sequencing (CITE-seq) on CD31+ FACS-isolated cells from the aorta-gonad-mesonephros (AGM) of E10.5 wildtype mouse embryos (Figure 1). CITE-seq can simultaneously label cells based on surface protein markers and profile single-cell transcriptomes, therefore bypassing the need for isolation of rare cells by FACS. Unsupervised clustering based on single cell transcriptomes showed distinct populations of EC (composed of venous and arterial subpopulations), cells undergoing EHT that expressed both endothelial and hematopoietic genes, and mature hematopoietic cells. In addition to EC and EHT clusters, the CITE-seq data revealed a small population of pre-hemogenic endothelial cells (pre-HE) that was distinct from HE cells, which clustered with "EHT" cells (Figure 1). Pre-HE cells expressed high levels endothelial markers and Cd44, a marker recently associated with hemogenic potential, but lacked expression of the prototypic HE markers Runx1 and Gfi1(Figure 1). To look for drivers of hematopoietic cell fate we identified 12 transcription factors (TFs) that were upregulated between the arterial endothelial cells (aEC) and pre-HE clusters identified by CITE-Seq, and that persisted through EHT. Within this list, Meis1 ranked as being the most significantly enriched, with its binding partner Pbx1 ranked fourth, therefore we hypothesized that Meis1 may be required to drive early hemogenic specification. We generated embryos with endothelial-specific deletion of Meis1 to investigate the functional requirement for Meis1 in EC. Endothelial-specific deletion of Meis1 impaired the formation of functional Runx1-expressing HE which significantly impeded the emergence of pre-HSPC via EHT.
In summary, we used a combination of CITE-seq and bulk RNA-seq to identify Meis1 as a candidate early functional marker of hemogenic specification. We show that Meis1 primes arterial endothelial cells towards the hemogenic trajectory, and that it is required in the mouse embryo for the formation of early pre-hemogenic cells prior to the emergence of functional HE and pre-HSPCs. Our findings implicate Meis1 in a critical fate-determining step for establishing EHT potential in endothelial cells.
Disclosures
Cole:AbCellera (Spouse): Current Employment. Coulombe:ExCellThera: Current Employment. Fentiman:StemCell Technologies: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal